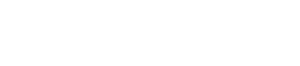Restore and Protect. Naturally.
Pericardial Integrity Matters
Rebuild the Natural Barrier1,2
- Reduce adhesions to surrounding tissue
- Preserve retrosternal distance for easier reentry
- Protect the heart from mechanical injury
Engineered for a Tension-Free, Hemostatic Closure2,3
- Suture-friendly for fast, secure fixation
- Conforms to minimize tension and bleeding at suture lines
- No stretching or forced approximation required. Enables full pericardial coverage— even after large resections
Support Better Surgical Outcomes1,2,4
- May reduce post-op complications and hospital readmissions
- Aids in identification of post-op bleeding
- Contributes to a cleaner operative field during reentry
ProxiCor is carefully trimmed to fit the defect precisely and sutured circumferentially to ensure secure placement and optimal tissue integration
Courtesy of Dr. Alfredo Rego
Fewer Post-Op Complications.
Naturally.
Pericardial closure with ProxiCor was shown to reduce post-operative complications in the RECON Study.
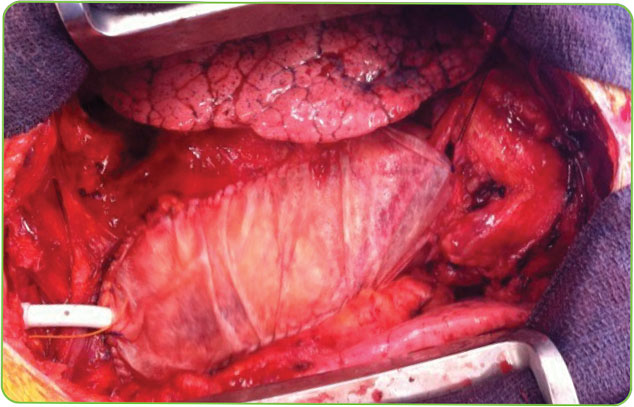
This prospective, multicenter study evaluated 1,420 patients undergoing cardiac surgery (CABG or valve repair/replacement). ProxiCor was used for pericardial closure following the index procedure, with outcomes compared to a matched national cohort.¹
The RECON Study
Included the most common cardiac surgeries
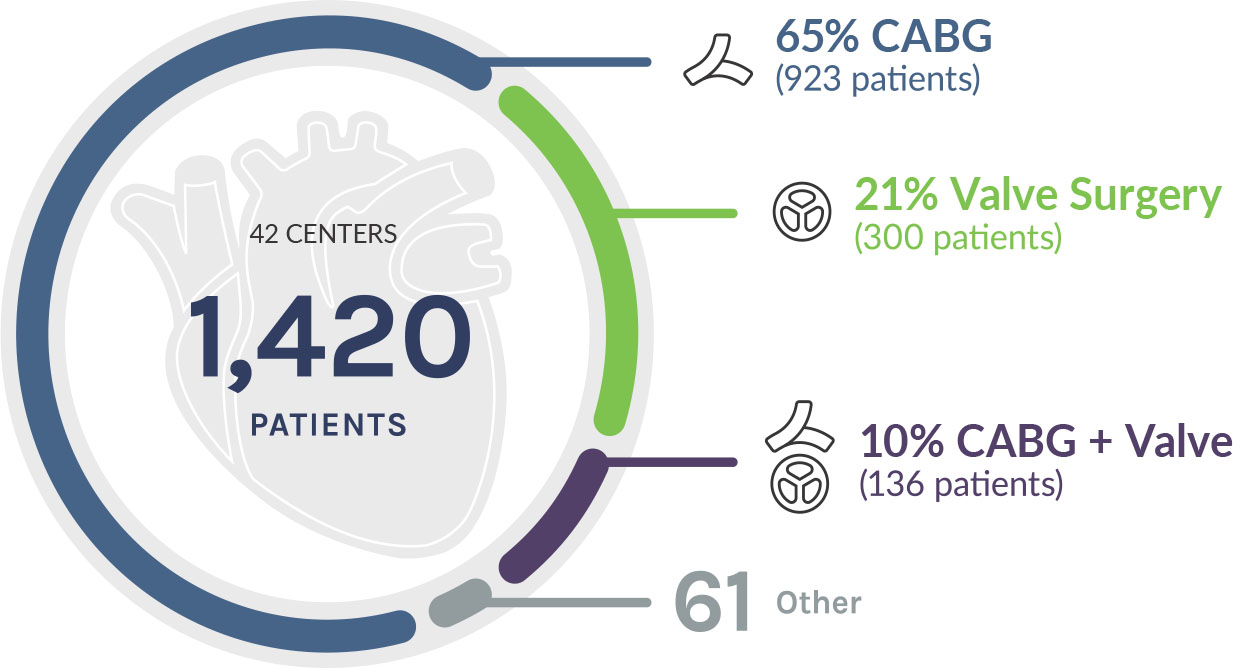
The benefits of closing
* p<0.05, statistically significant
Elutia’s Biomatrices for Cardiovascular Tissue
The Natural Choice for Repair and Regeneration
Elutia’s biomatrix portfolio is built on engineered extracellular matrix (ECM) biology—designed to facilitate surgical repair, support healing, and address key causes of complications.
Trusted in over one million patients and supported by hundreds of peer-reviewed publications, Elutia’s ECM biomatrices offer confidence in every repair.
Naturally Better
Unlike other products that are crosslinked—compromising their ability to remodel—Elutia’s biomatrices preserve what matters for healing.

Clinical Experience in Infected and High-Risk Surgical Fields*
Elutia’s biomatrices (ProxiCor, VasCure) have been used for valve and annular repair in patients with endocarditis, as well as in high-risk conditions such as prior infection or radiation. Though based on limited number of patients, these cases showed no infection-related failures and good tissue integration—supporting potential use in contaminated or compromised fields.7,9,10
*ProxiCor, VasCure and Tyke are not specifically cleared or indicated by regulatory authorities for the prevention or treatment of infection. Clinical judgment should guide their use in infected or high-risk surgical fields.
Trusted by surgeons. Validated by data. Naturally.
1. Rego A, et al. J Cardiothorac Surg. 2019;14(1):61.
2. Rego A, et al. Heart Surg Forum. 2022;25(1):E008–E019.
3. Sundermann S, et al. Interact Cardiovasc Thorac Surg. 2015;20(1):10–14.
4. Rao V, et al. Ann Thorac Surg. 1999;67(2):484–488.
1. Rego A, et al. J Cardiothorac Surg. 2019 Mar 15;14(1):61.
2. Kaleda VI et al. Interact Cardiovasc Thorac Surg. 2012;14:384–9;
3. Saxena A et al. Am J Cardiol. 2012;109:219–25;
4. Rostagno C et al. J Cardiothorac Vasc Anesth. 2010;24:952–8;
5. Filardo G et al. Circ Cardiovasc Qual Outcomes. 2009;2:164–9;
6. Aranki SF et al. Circulation. 1996;94:390–7;
7. Mathew JP et al. JAMA. 2004;291:1720;
8. El-Chami MF et al. J Am Coll Cardiol. 2010;55:1370–6;
9. Helgadottir S et al. J Cardiothorac Surg. 2012;7:87;
10. Shen J et al. J Thorac Cardiovasc Surg. 2011;141:559–70.
1. Allen K, et al. Front Cardiovasc Med. 2021;8:631750.
2. Bibevski S, et al. Front Cardiovasc Med. 2020;7:562136.
3. Ferng A, et al. Ann Thorac Surg. 2017;104(3):e239–e241.
4. Grimes M, et al. Biomed Mater Eng. 2005;15(1–2):65–71.
5. Haney L, et al. Ann Thorac Surg. 2021;S0003-4975(21)01384 undermann S, et al. Interact Cardiovasc Thorac Surg. 2015;20(1):10–14.
8. Elutia Data on File.
9. Myers P, et al. Circulation. 2012;126:A16130.
10. Gerdisch M, et al. J Thorac Cardiovasc Surg. 2014;148(4):1370–1378.
11. Badylak S, et al. J Biomed Mater Res B Appl Biomater. 2003;67(1):648–654.
12. Jernigan T, et al. Ann Surg. 2004;239(5):733–738.
WMK-1503-01F