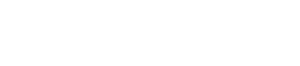Engineered for Durable Repair. Naturally.
VasCure combines the strength to withstand arterial pressures with the flexibility to conform to complex anatomies—supporting durable repair and healing where it matters most.
Designed for Complex Vascular Repairs1,3
- Supports vascular integrity while maintaining compliance
- Conforms to challenging anatomies for precise repairs
- Provides mechanical strength to reinforce suture lines and withstand vessel pressures
Clinically Backed Performance1
- Enhances healing and recovery at the repair site
- Reduces the risk of restenosis, thrombosis, and other complications
- Promotes site-specific tissue remodeling and natural integration
- Promotes sustained vessel performance over time
- Helps minimize the need for reintervention
A versatile solution for vascular repair procedures, including:

Whether repairing the carotid or femoral artery, reinforcement must withstand arterial pressure and movement without compromising healing. VasCure provides the strength for repair and the flexibility to conform to complex anatomies—ideal for endarterectomy closure and beyond.
Clinical Evidence at a Glance:
Proven Safety and Performance¹
VasCure delivers durable, healthy healing across a range of vascular repairs
- ~100% procedural success in iliofemoral and carotid artery cases
- Excellent vessel patency up to 24 months post-op
- <3% restenosis rate
- <0.8% graft-related adverse events
Real-World Integration
A 55-year-old male underwent femoral endarterectomy with VasCure patch angioplasty. Over three procedures spanning 19 months, two separate patches were observed to be fully integrated and visually indistinguishable from native artery.
Histology confirmed site-specific remodeling with no inflammation or degeneration. Post-operative testing showed normal function and complete pain symptom resolution. (See Figures, below)
“Synthetic materials, such as polyethylene terephthalate (Dacron®) or polytetrafluoroethylene (PTFE), are ready to use and have a long shelf-life, have high biomechanical strength, but do not mimic the native vasculature, and stimulate a foreign body response on implantation which can lead to post-operative complications due to chronic inflammation, lack of remodeling, limited compliance, and poor resistance to infection.”
—Allen K, et al. Front Cardiovasc Med. 2021
Complete Integration in Femoral Artery Repair1
PERFORM Study: 221 patients with iliofemoral artery repair
Carotid Registry: 45 cases of carotid artery reconstruction (38 patients)
Figure 1:
Initial patch angioplasty of the right common femoral artery (CFA) using VasCure (arrow) following endarterectomy.
Figure 2:
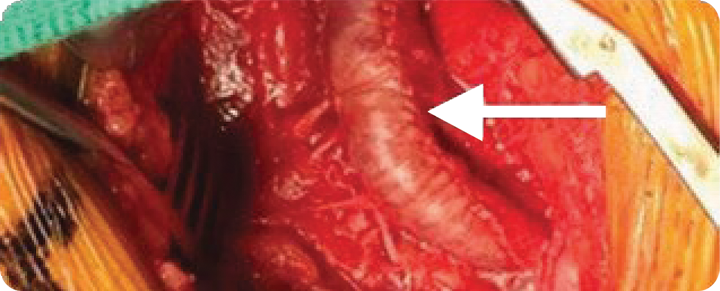
At 16 months, the VasCure patch (arrow) in the right CFA was fully incorporated into native vascular tissue, indistinguishable from the surrounding artery. Due to disease at an adjacent site, a second VasCure patch was used to extend the repair.
Figure 3:
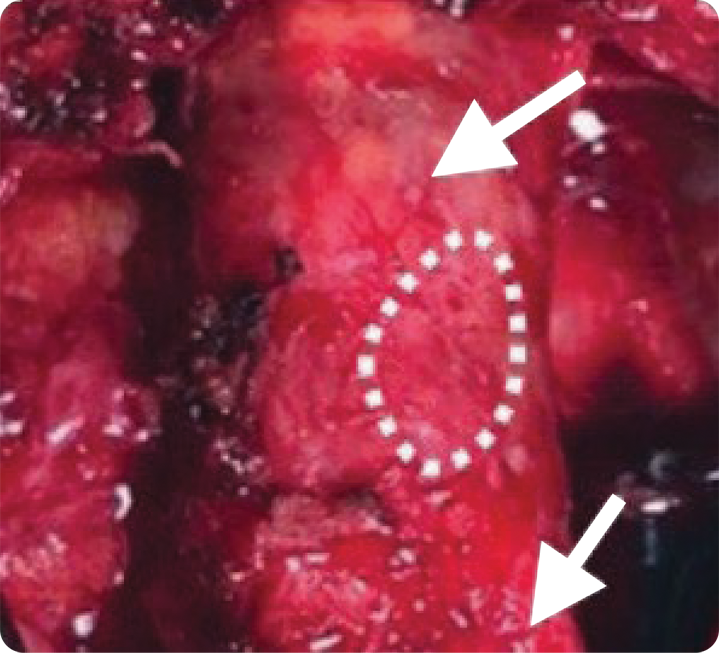
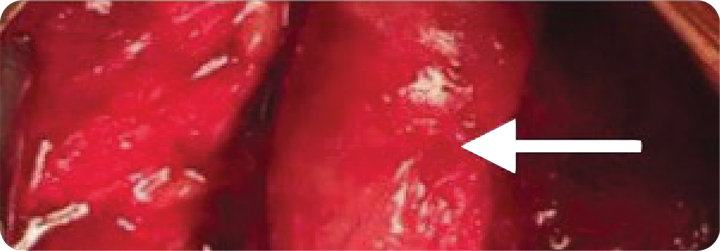
Twenty months post-index procedure, a third surgery was performed for bypass. Both previously placed VasCure patches (arrows) appeared fully incorporated into the native femoral artery. The dashed line indicates the patch-to-artery anastomosis for histology.
Elutia’s Biomatrices for Cardiovascular Tissue
The Natural Choice for Repair and Regeneration
Elutia’s biomatrix portfolio is built on engineered extracellular matrix (ECM) biology—designed to facilitate surgical repair, support healing, and address key causes of complications.
Trusted in over one million patients and supported by hundreds of peer-reviewed publications, Elutia’s ECM biomatrices offer confidence in every repair.
Naturally Better
Unlike other products that are crosslinked—compromising their ability to remodel—Elutia’s biomatrices preserve what matters for healing.

Clinical Experience in Infected and High-Risk Surgical Fields*
Elutia’s biomatrices (ProxiCor, VasCure) have been used for valve and annular repair in patients with endocarditis, as well as in high-risk conditions such as prior infection or radiation. Though based on limited number of patients, these cases showed no infection-related failures and good tissue integration—supporting potential use in contaminated or compromised fields.7,9,10
*ProxiCor, VasCure and Tyke are not specifically cleared or indicated by regulatory authorities for the prevention or treatment of infection. Clinical judgment should guide their use in infected or high-risk surgical fields.
Trusted by surgeons. Validated by data. Naturally.
1. Piterina A, et al. Int J Mol Sci. 2009 Nov 20;10(10):4375-4417.
2. Ferng A, et al. Ann Thorac Surg. 2017 Sep;104(3):e239-e241.
3. Grimes M, et al. Biomed Mater Eng. 2005;15(1-2):65-71.
4. Slachman FN. Ann Thorac Surg. 2014;97(5):e129.
1. Allen K, et al. Front Cardiovasc Med. 2021;8:631750.
2. Bibevski S, et al. Front Cardiovasc Med. 2020;7:562136.
3. Ferng A, et al. Ann Thorac Surg. 2017;104(3):e239–e241.
4. Grimes M, et al. Biomed Mater Eng. 2005;15(1–2):65–71.
5. Haney L, et al. Ann Thorac Surg. 2021;S0003-4975(21)01384 undermann S, et al. Interact Cardiovasc Thorac Surg. 2015;20(1):10–14.
8. Elutia Data on File.
9. Myers P, et al. Circulation. 2012;126:A16130.
10. Gerdisch M, et al. J Thorac Cardiovasc Surg. 2014;148(4):1370–1378.
11. Badylak S, et al. J Biomed Mater Res B Appl Biomater. 2003;67(1):648–654.
12. Jernigan T, et al. Ann Surg. 2004;239(5):733–738.